While advancements in medicine have proven to be integral in improving quality of care in the U.S., these innovations must be approved for clinical use. The Food and Drug Administration is responsible for determining the efficacy and safety of products before they are introduced to the national market.
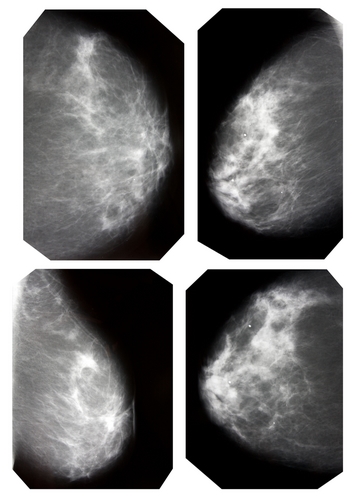
According to MarketWatch, the FDA recently authorized the intravenous use of Gadavist from Bayer HealthCare in MRI scans of the breast to determine the presence of malignant growths. The approval is based on a review of two multi-center phase III studies that were conducted in 13 countries. While American health care has made considerable strides in the detection and treatment of breast cancer, one in five women still have the disease go undetected after undergoing a mammogram.
“This is an important diagnostic tool for health care professionals with breast cancer patients. Breast MRI with Gadavist provides important visibility for assessment of malignant breast disease and for treatment planning,” said Gillian Newstead M.D., GEMMA Principal Investigator, from the University of Chicago Medical Center, quoted by MarketWatch.
The quick approval from the FDA falls in line with recent efforts from diagnostic imaging societies that encourage improved capabilities, such as the Radiological Society of North America and the American College of Radiology.
The path of Gadavist
Originally, the compound was approved back in March 2011 for IV use in diagnostic MRI in adults and children aged 2 years and older. Gadavist was intended to detect and visualize disruptions to the blood brain barrier or abnormal vascularity of the central nervous system, Diagnostic Imaging reported.
The clinical trials included 787 patients who were recently diagnosed with breast cancer. Each of the MRI exams were reviewed by three separate radiologists. The researchers found that breast MRIs that were enhanced with Gadavist demonstrated increased sensitivity (80 to 89 percent) to the presence of malignant diseases, compared to unenhanced MRI (37 to 73 percent). However, it was noted that some of Gadavist MRIs overestimated the extent of the diseased breast in roughly half of the patients.
To offset the MRIs, three additional radiologists conducted interpretations on x-ray mammography alone. Between the two studies, sensitivity for the presence and extent of malignancy ranged from 68 to 73 percent, while specificity ranged from 86 to 84 percent in breasts lacking any abnormalities.
According to the U.S. Centers for Disease Control and Prevention, breast cancer is the most common cancer among women in the world. But the development of Gadavist-enhanced digital imaging could lead to improved detection and treatment of malignancies, improving care strategies and health outcomes as a result.
Contact Viztek for more information.
Ronny Bachrach
Latest posts by Ronny Bachrach (see all)
- Konica Minolta Debuts First-of-Its-Kind Digital U-Arm System at AHRA - July 27, 2016
- Researchers Detect Signs Of Stroke Risk Using MRI - June 27, 2016
- Imaging Biz: Q&A with David S. Channin MD: How to Make PACS Patient Centered - June 22, 2016